Function of Type VI secretion systems: roles and toxins
The T6SS is considered as a versatile machinery due to its ability to inject toxins to either prokaryotic or eukaryotic cells, to mediate interbacterial interactions or pathogenesis.
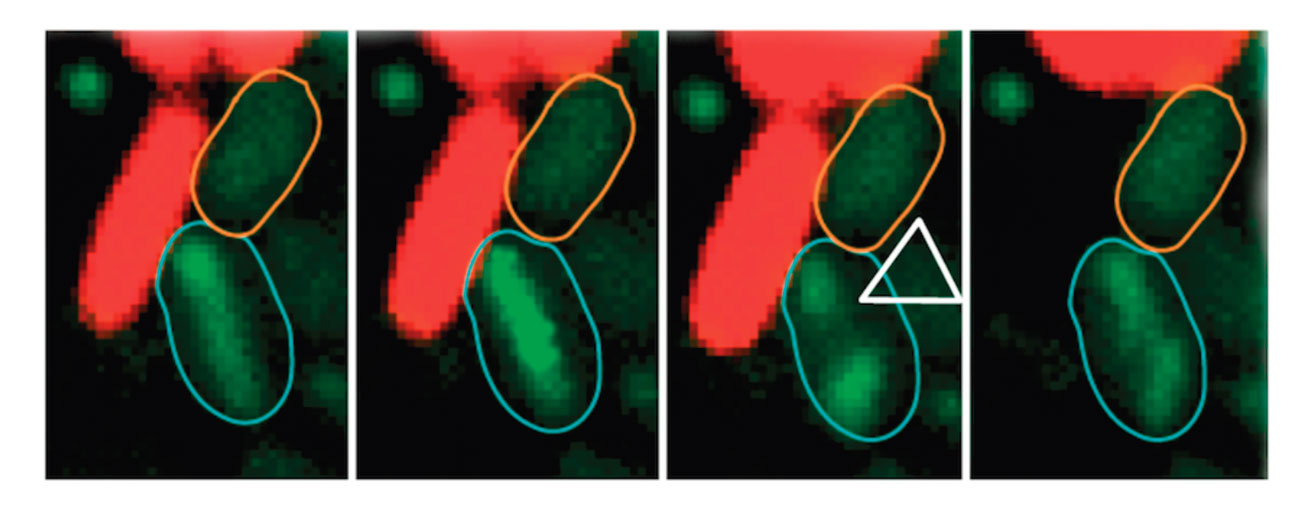
We have reported that the EAEC Sci-1, Sci-2, the Citrobacter rodentium CTS-1 and Salmonella enterica Typhimurium T6SSs have anti-bacterial activities (Brunet et al., 2013) (Gueguen et al., 2013) (Brunet et al., 2015) (Flaugnatti et al., 2016).
A large number of T6SS effectors with antibacterial activities have been characterized. Cell-wall targeting effectors called Tae (type VI secretion amidase effector) and Tge (type VI secretion glycoside hydrolase effector) degrade the peptidoglycan of target cells. Membrane targeting effectors like Tle (Type VI lipases effectors) hydrolyse the membrane phospholipids of the target cells. Finally, effectors with nuclease activity (Tde family) have also been identified. These antibacterial effectors are produced concomitantly with specific immunity proteins to prevent self-intoxication.
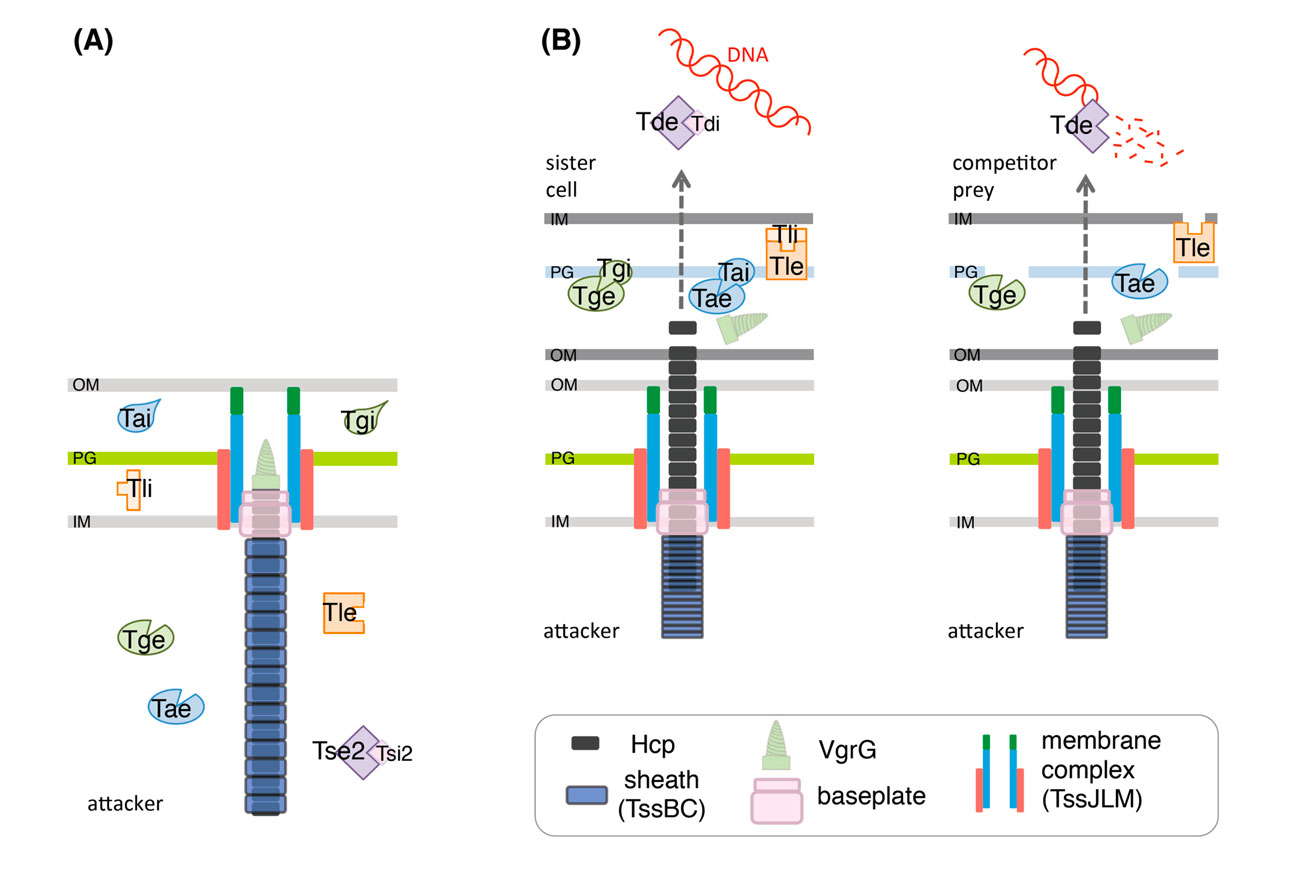
Schematic representation of the T6SS in the extended (A) and contracted (B) conformations. A selection of characterized effector proteins (Tae, Tge, Tle and Tde), and of their cognate immunity proteins (Tai, Tgi, Tli and Tdi, respectively) is shown. In a sister cell, the activity of the effector proteins will be counteracted by the cognate immunity protein (B, left) whereas if the prey cell is an unrelated species/strain, the effector proteins are delivered into the periplasm or the cytoplasm of the competitor to degrade the target molecule (cell wall for Tae and Tge; membrane lipids for Tle; DNA for Tde) (B, right). (Durand et al., 2014).
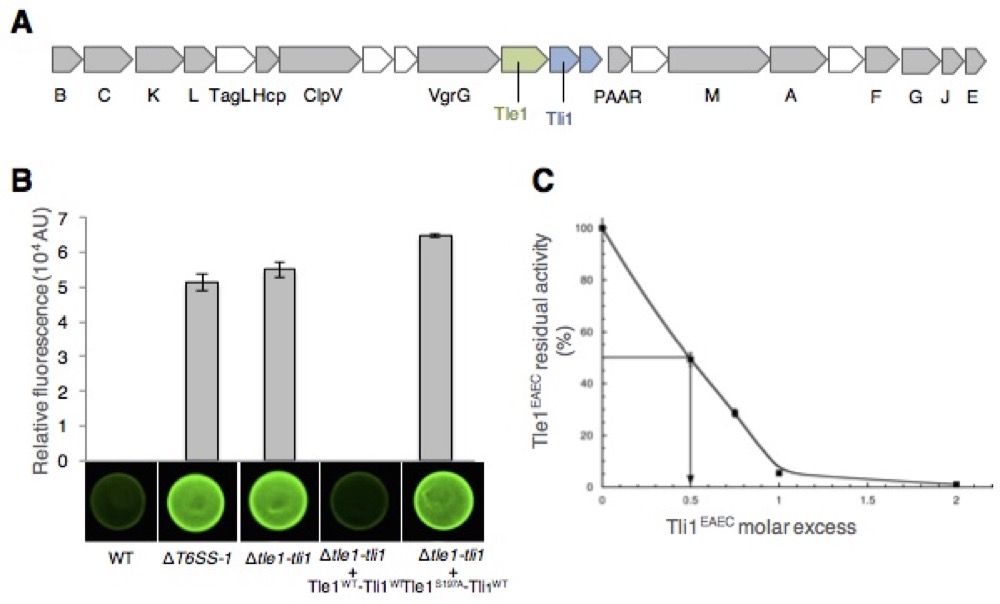
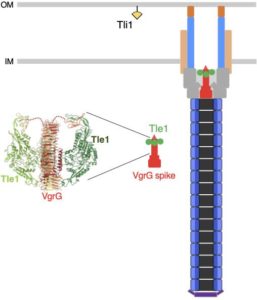
Nicolas showed that Sci-1 is involved in interbacterial competition and identified Tle1/Tli1, a toxin/immunity couple encoded downstream the vgrG1 gene. In collaboration with the group of Stéphane Canaan, Nicolas showed that Tle1 is a periplasmic acting toxin with phospholipase A1 and A2 activities and responsible for the inter-bacterial activity mediated by the Sci-1 T6SS. Self-protection of the attacker cell is secured by Tli1, an outer membrane immunity lipoprotein that binds tightly to Tle1 to block its phospholipase A1 activity (Flaugnatti et al., 2016).
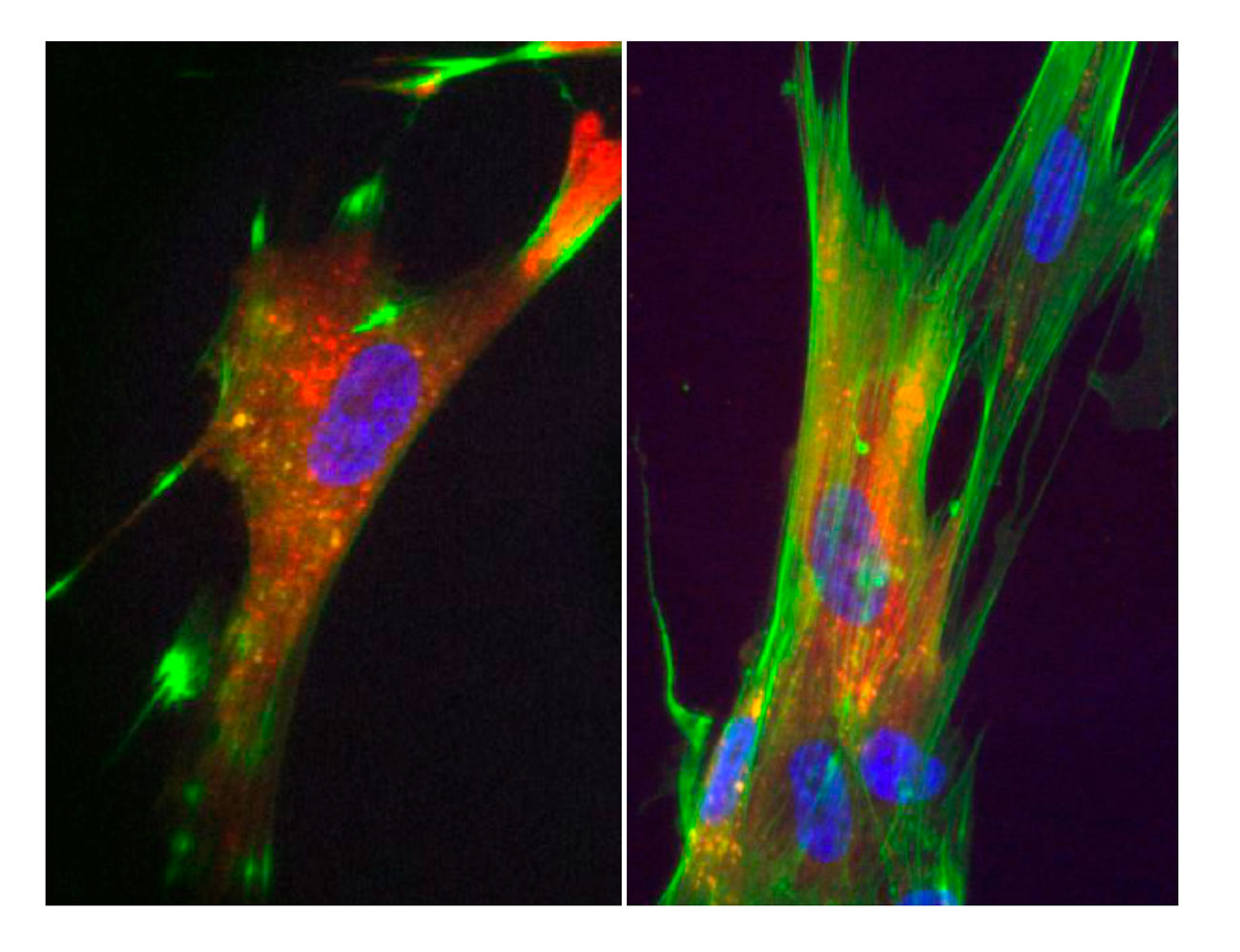
In addition to anti-bacterial effectors, several anti-host effectors have been characterized. These toxins can be either independent or fused to the C-terminal of VgrG protein, the T6SS puncturing device. For example, the toxin carried by VgrG1 of V. cholerae catalyses the covalent cross-linking of G-actin in an ATP-dependent manner. This activity prevents host cell cytoskeleton rearrangements and thus protects V. cholerae from phagocytosis by macrophages. In collaboration with the group of Christian Cambillau (AFMB) and Gilles Audoly, Eric purified the C-terminal domain of VgrG1 (CTD) and found that the protein is active to cross-link actin and causes actin dynamic curbing when injected in macrophages or fibroblasts.
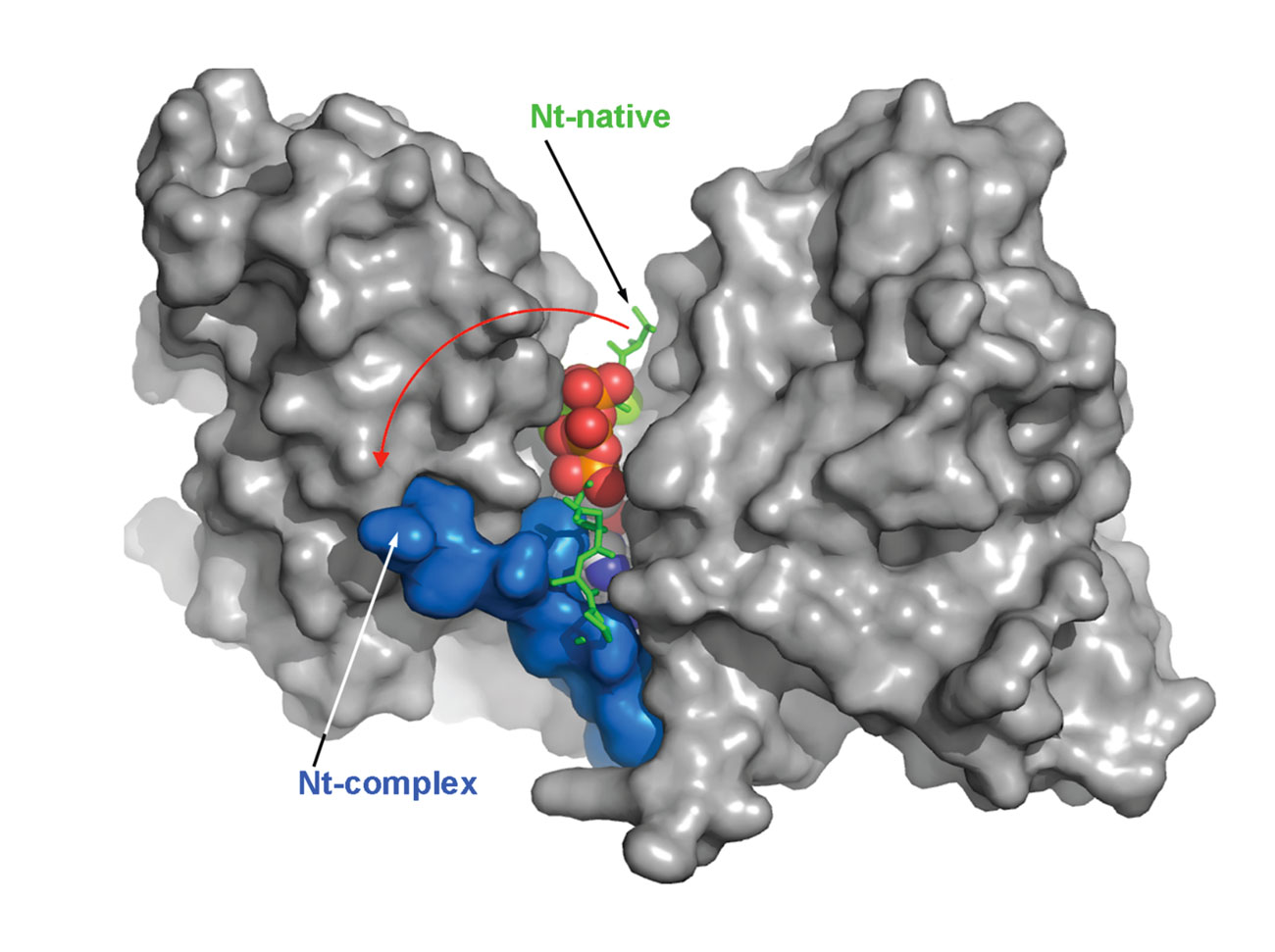
The crystal structure of the VgrG1 CTD was solved. The structure exhibits a V-shape, with the catalytic site located at its base. The N-terminal domain that seals the active site is displaced upon ATP binding, rendering the active site available for actin binding and cross-linking (Durand et al., 2012).