The Type VI secretion system
The main theme of research in our laboratory is dedicated to accumulate information on the Type VI secretion system. We initiated the work on this topic in January 2008 using a pathogenic strain of Escherichia coli, named enteroaggregative E. coli (EAEC) as model microorganism. This group of strains is an emerging pathotype responsible for severe and persistent diarrhea of infants, young children, or immunocompromised individuals. Although not well understood, the EAEC virulence mechanism is based on colonization of the intestinal mucosa involving both the flagella and adhesive structures called fimbriae, formation of a thick biofilm at the surface of the colon epithelia, and secretion of entero- and cytotoxins.
The Type VI secretion system – or T6SS – is a multi-protein secretion machine that delivers toxin proteins, in a contact-dependent manner, directly into target cells. The T6SS is versatile as it can target both eukaryotic and prokaryotic cells, specifically delivering toxins that have actin cross-linking (eukaryotic targets), peptidoglycan hydrolase (prokaryotic targets) or phospholipase or DNase (both eukaryote and prokaryote targets) activities. Anti-host activities have been shown to inhibit phagocytosis or to destroy macrophages, while the anti-bacterial activities allow the bacterium to destroy competitors and to have a privileged access to the niche, to nutrients, or to new DNA.
The machine is constituted of 14 different proteins, encoded by genes that are usually clustered (Fig. 1). The 14 proteins assemble two complexes: a complex structurally and functionally similar to the tail of contractile phages, anchored to the cell envelope by the membrane complex.

Fig. 1. Schematic representation of the T6SS genes. The co-occurrence between the genes (adapted from Boyer et al., 2009) and the sub-cellular localization of the corresponding proteins are indicated (cyto, cytoplasm ; IM, inner membrane; OM, outer membrane). The localization and topology of membrane proteins are indicated, as well as the homologies between T6SS and phage components (Publication).
The membrane complex is composed of three components : the TssL and TssM inner membrane proteins and the TssJ outer membrane lipoprotein (Fig. 1 & 2). These proteins are connected through interactions between TssM and TssL, and TssM and TssJ. This trans-envelope complex is a structural determinant of the T6SS as it anchors the phage tail-like structure to the membrane, but is also probably dynamic as conformational changes of TssM have been evidenced. Usually the TssL protein or an accessory protein named TagL, possesses a periplasmic extension that binds to the peptidoglycan layer and stabilizes the complex.
The phage tail-like complex is composed of subunits that share structural and functional similarities with the components of the tail of contractile phages (Fig. 1 & 2). The Hcp protein forms hexameric rings that stack on each other to build a tubular structure similar to the inner tube of phage. This Hcp tube is wrapped by the TssBC proteins that act similarly to the phage sheath. They assemble in a metastable, extended conformation that stores mechanical energy that is released to contract the structure and propel the inner tube. The tube is tipped by the VgrG protein, which is similar to the gp27 :gp5 complex, the spike that is necessary to perforate the target cell. The tail tube/sheath assemble on an assembly plateform called the baseplate of unknown composition.
In the extended conformation, the T6SS tail reaches several hundreds of nanometers and has been observed in V. cholerae cells (Fig. 2A). Based on these cryo-electromicrographs, the homologies with bacteriophage proteins and the current information on protein localization, protein-protein interactions, a model has been proposed (see Fig. 2C)
![Fig. 2. T6SS architecture. (A) Cryo-electron micrograph highlighting the presence of a micrometer-long tubular structure in the cytoplasm of a Vibrio cholera cell )(after Basler et al., 2012). (B) the magnification highlights the putative sub-complexes (yellow, membrane complex ; pink, baseplate ; green, tail tube/sheath)(after Basler et al., 2012). (C) Schematic representation of the T6SS architecture (OM, outer membrane; PG, peptidoglycan; IM, inner membrane). (from [Publication 9-21])](http://cascaleslab.fr/wp-content/uploads/2015/01/T6SSG-fig2.jpg)
Fig. 2. T6SS architecture. (A) Cryo-electron micrograph highlighting the presence of a micrometer-long tubular structure in the cytoplasm of a Vibrio cholera cell )(after Basler et al., 2012). (B) the magnification highlights the putative sub-complexes (yellow, membrane complex ; pink, baseplate ; green, tail tube/sheath)(after Basler et al., 2012). (C) Schematic representation of the T6SS architecture (OM, outer membrane; PG, peptidoglycan; IM, inner membrane). (Publication)
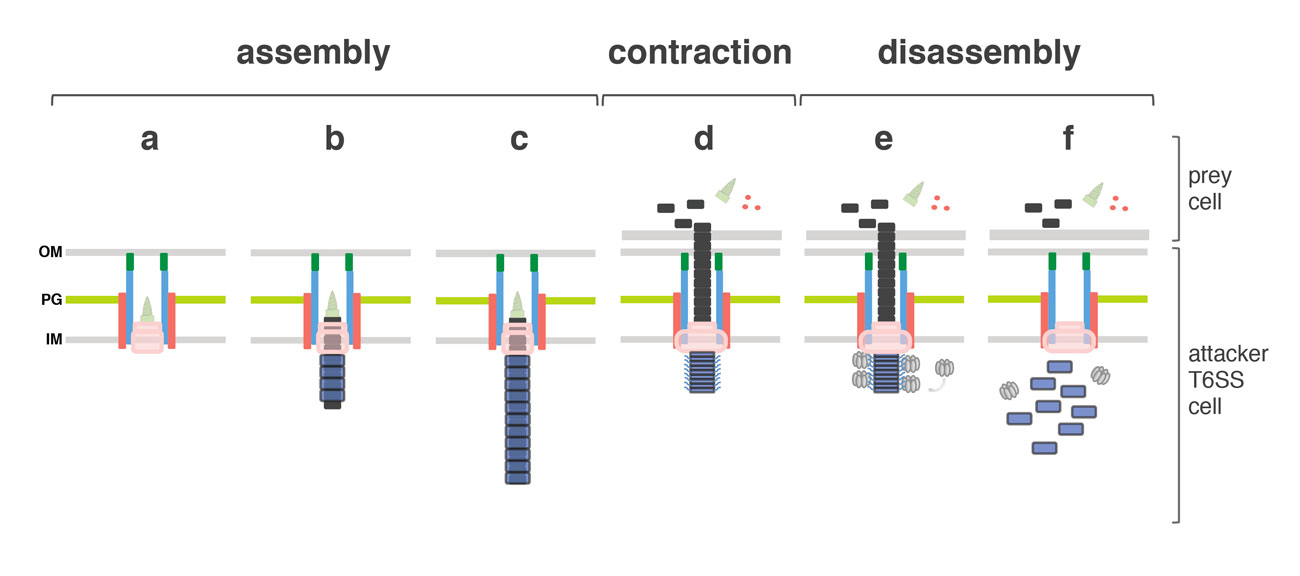
Fig. 3. Schematic model of the T6SS mechanism of action. From left to right : assembly of the T6SS starting with the membrane complex, the baseplate structure (a) and the tail (b,c), contraction of the sheath, target cell membrane perforation and effector delivery (d), recruitment of ClpV (e) and sheath recycling (f).
The main questions we are addressing are how the membrane complex assembles in the cell envelope ? How the tail-like structure assembly is controled ? How the presence of the target cell is signaled to the T6SS ? We are also interested in additional questions regarding the T6SS regulatory mechanisms and effectors.
If you want to know more on our contribution in the field, please visit the corresponding webpages. We have first focused our attention on the assembly of this multi-protein secretion system. However, we are always interested in the regulatory mechanisms that control the expression of the gene clusters encoding the T6SS. More recently, we have developed a new line of study aimed to identify and characterize the toxin effectors that are delivered by this secretion apparatus and how the effectors are recruited and transported.
We also recently initiated a new topic of research on a novel secretion machine, the Type IX secretion system.

